Cloning vectors in 2025: from genes to genomes
|
|
Time to read 10 min
|
|
Time to read 10 min
Cloning vectors are still essential in 2025.
Classic plasmids shaped modern cloning.
Homology-based assembly is replacing MCSs.
New selection systems are needed beyond antibiotics.
Smaller, tunable vectors will drive the future.
Cloning vectors are one of the foundational tools of molecular biology. They are engineered DNA molecules that serve as vehicles to propagate, manipulate, and analyze genetic material. The emergence of cloning vectors in the 1970s transformed biology from a descriptive science into one capable of engineering the genetic makeup of living organisms. Over time, these vectors have evolved to handle increasingly ambitious tasks: from inserting a single gene into E. coli to maintaining entire viral genomes.
In 2025, cloning vectors remain indispensable. Advances in gene synthesis, genome editing, and synthetic biology may have reduced the need for traditional restriction–ligation cloning, but vectors continue to anchor the workflows that connect digital sequence designs with living systems. This article provides a comprehensive overview of cloning vectors, tracing their history, highlighting their key families, and exploring their modern applications in research and biotechnology.
The story of cloning vectors begins in the early 1970s with the first recombinant DNA experiments. Scientists like Paul Berg, Herbert Boyer, and Stanley Cohen pioneered techniques to cut DNA with restriction enzymes and join it with plasmids that could replicate inside bacteria. These breakthroughs were controversial at the time, leading to the famous Asilomar Conference in 1975, but they laid the foundation for modern biotechnology.
The earliest workhorse vectors were derivatives of naturally occurring plasmids such as ColE1. Researchers modified these plasmids to carry antibiotic resistance genes, which provided selectable markers, and to contain multiple restriction sites, which enabled the insertion of foreign DNA. One of the most influential of these was pBR322, developed in 1977. It contained resistance to both ampicillin and tetracycline, a ColE1 origin of replication, and a set of convenient restriction sites.
As the field matured, new vector families were engineered to improve upon pBR322. The pUC series, introduced in the 1980s, offered higher copy numbers and incorporated the lacZ α-complementation system for blue-white screening. This system became the backbone of countless cloning projects in academic labs and biotech companies.
The subsequent decades brought diversification: specialized vectors were designed for sequencing, protein expression, shuttle functions across multiple species, and large DNA maintenance. Each generation reflected the evolving ambitions of molecular biology.
When most biologists think of cloning vectors, they imagine plasmids used to isolate, propagate, and manipulate single genes. These vectors share several key design principles:
Origin of replication (ori): Determines how many copies of the plasmid are present in each bacterial cell. High-copy origins (e.g., pUC) yield large amounts of DNA, while low-copy origins provide greater stability for inserts that may be toxic.
Selectable markers: Usually, antibiotic resistance genes (ampicillin, kanamycin, chloramphenicol, tetracycline). They ensure that only bacteria carrying the plasmid survive.
Multiple cloning site (MCS): A short synthetic sequence containing many unique restriction sites, enabling researchers to insert DNA fragments flexibly.
Reporter genes: Systems like lacZ α allow visual screening of successful recombinants.
The pUC vectors remain iconic in cloning history. They are small (~2.7 kb), carry an ampicillin resistance gene, and have a high-copy ColE1-derived origin. Their blue-white screening system using lacZ was revolutionary, enabling researchers to quickly identify colonies containing inserts. Over the years, many derivatives were created to suit different cloning needs:
pUC18 and pUC19 – The original pair. Both carry the lacZ α fragment for blue-white screening but differ in the orientation of the multiple cloning site (MCS). This gave researchers flexibility depending on restriction enzyme availability.
pUC118 and pUC119 – Adapted for sequencing with the M13 phage system. These vectors contain an f1 origin of replication, allowing production of single-stranded DNA for Sanger sequencing.
pUC57 – A popular derivative widely used for gene synthesis. Its small size and versatile MCS make it an efficient backbone for synthetic DNA fragments.
Other pUC variants – Numerous specialized versions exist, optimized with different promoters, tags, or antibiotic markers, but all trace back to the same streamlined, high-copy design.
Together, the pUC family set the standard for what a “modern” cloning plasmid should look like: compact, versatile, and highly adaptable.
pBluescript, released by Stratagene in the late 1980s, became another standard. It included features optimized for sequencing and in vitro transcription, and could be propagated in both E. coli and other systems. Its versatility made it a staple of many cloning projects. These plasmids include a Dual promoter system (T7 and T3) – Each vector includes promoters flanking the MCS, enabling in vitro transcription from either strand. This feature made pBluescript especially valuable for generating RNA probes and sequencing templates. They also include a f1 origin of replication – Allows production of single-stranded DNA, supporting phage display and Sanger sequencing workflows.
The family includes several well-known derivatives:
pBluescript KS (+/–) – The most common versions. “KS” indicates the KpnI–SacI region within the multiple cloning site (MCS). Available in both orientations (+ and –), giving flexibility in insert direction relative to promoters.
pBluescript SK (+/–) – Similar design, but the MCS spans the SacI–KpnI region (“SK”). Also available in both orientations, making it easy to clone and express fragments in either direction.
The availability of multiple orientations, dual promoters, and an expanded MCS made pBluescript a Swiss-army-knife vector in the pre-synthesis era, widely used in mapping, sequencing, and probe generation.
pGEM vectors, developed by Promega, became popular in the 1990s for their convenience in PCR cloning and probe generation. Like pUC and pBluescript, they are small, high-copy plasmids carrying an ampicillin resistance marker and a lacZ α-fragment for blue-white screening. Their main representatives are:
pGEM-3Z and pGEM-4Z – Early versions with expanded multiple cloning sites and flanking promoters (SP6 and T7) for in vitro transcription.
pGEM-T – Designed for TA cloning of PCR products. Takes advantage of Taq polymerase’s 3′ A overhangs, allowing direct ligation into T-tailed vectors.
pGEM-T Easy – A simplified, higher-efficiency derivative of pGEM-T, with optimized vector size and ligation performance.
Together, the pGEM family made PCR product cloning faster and more reliable, cementing their place as a go-to vector series for everyday molecular biology.
While pUC, pBluescript, and pGEM relied on multiple cloning sites and restriction–ligation, the Gateway system introduced a recombination-based approach. Derived from the bacteriophage λ integration/excision mechanism, Gateway vectors use att recombination sites to transfer DNA fragments between plasmids with high efficiency.
Entry vectors – DNA fragments are first cloned into a “donor” vector using BP recombination.
Destination vectors – The insert can then be shuttled into a wide variety of expression or functional plasmids using LR recombination.
Advantages – Highly efficient, no need for restriction sites, scalable for parallel cloning.
Applications – Widely used in functional genomics, high-throughput screening, and expression studies.
Gateway didn’t fully replace traditional cloning vectors, but it became a dominant technology in the 2000s, especially for large projects where the same insert needed to be tested in multiple vector contexts.
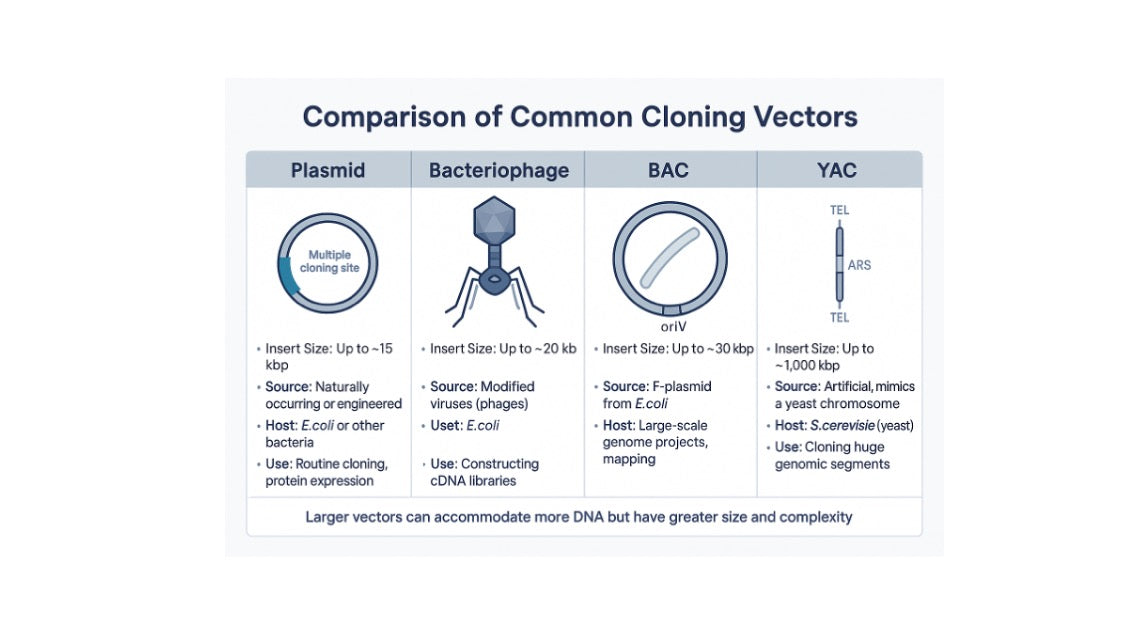
As molecular biology matured, scientists faced the challenge of cloning DNA fragments far larger than a single gene. Sequencing the human genome or reconstructing viral genomes required vectors capable of carrying hundreds of kilobases of DNA. Several major vector classes emerged to meet these needs.
Cosmids are hybrids of plasmids and bacteriophage λ DNA. They can accommodate inserts up to ~45 kb, much larger than standard plasmids. They were widely used in the 1980s for constructing genomic libraries. However, their use has declined as newer vector systems have become available.
Introduced in the 1980s, YACs could carry inserts of hundreds of kilobases to more than a megabase. They mimic natural yeast chromosomes, containing centromeres, telomeres, and origins of replication. YACs were instrumental in the early stages of genome mapping projects, but they suffered from instability and chimerism, leading to their replacement by BACs.
BACs became the workhorse of the Human Genome Project in the 1990s. Based on the E. coli F-factor plasmid, BACs maintain inserts of 100–300 kb with high stability. Their low copy number reduces recombination events, making them reliable for large-scale sequencing. Even in 2025, BACs remain relevant for synthetic biology, transgenesis, and the study of large viral genomes.
Even in the age of DNA synthesis, cloning vectors retain critical roles:
Gene library construction – Genomic or cDNA libraries still rely on cloning vectors (plasmids, cosmids, BACs) to maintain collections of DNA fragments for screening and analysis.
Genome sequencing and mapping – BACs and similar vectors enabled the Human Genome Project, and they remain useful in assembling reference genomes or difficult genomic regions.
Viral reverse genetics – Entire viral genomes can be cloned into vectors to enable manipulation, rescue of infectious virus, and vaccine development.
Synthetic biology platforms – Cloning vectors are used to assemble modular genetic parts, store intermediate constructs, and ensure traceability of designs.
Cloning vectors have carried molecular biology from the first recombinant DNA experiments to today’s era of synthetic biology. Their journey from historic plasmids like pBR322 to large BACs and YACs mirrors the expansion of biology’s ambitions — from cloning single genes to capturing entire genomes.
In 2025, cloning vectors remain indispensable, even as DNA synthesis has become routine. All sequence-verified synthetic DNA still requires cloning into a stable vector. Far from being obsolete, cloning vectors are the backbone of modern gene synthesis pipelines.
Yet their design is evolving. Traditional multiple cloning sites (MCS), once the hallmark of a good vector, are losing importance. Homology-based assembly methods such as Gibson assembly and NEBuilder make it possible to clone constructs without relying on pre-defined restriction sites. This flexibility is reshaping how vectors are used and designed.
Several challenges remain unsolved:
Selection markers: Antibiotic resistance genes have served well for decades, but their limitations are increasingly apparent, both from biosafety and regulatory standpoints. New strategies for selection — metabolic markers, toxin–antitoxin systems, or orthogonal resistance — are needed.
Origins of replication: Copy number is often fixed, but research would benefit from vectors whose replication can be tuned dynamically.
Vector size and efficiency: Smaller vectors reduce metabolic burden on host cells and increase the available “cargo” space for synthetic constructs.
Looking forward, the next generation of cloning vectors will be leaner, smarter, and more tightly integrated with digital design and synthesis workflows. They will not only carry DNA but actively shape how genetic designs move from computer screens to living cells.
The story of cloning vectors, once about blue-white colonies on agar plates, is now about scaling gene synthesis, engineering entire genomes, and enabling synthetic biology at industrial scale. As the field advances, cloning vectors will continue to evolve — remaining the invisible but essential backbone of biotechnology.
Cloning vector – A DNA molecule (usually a plasmid) engineered to carry and propagate foreign DNA in a host organism.
Origin of replication (ori) – DNA sequence that enables a plasmid to replicate inside a host cell, controlling its copy number.
Selectable marker – A gene (often conferring antibiotic resistance) that allows only cells containing the vector to grow.
Multiple cloning site (MCS) – A short engineered DNA region with many unique restriction sites, used for inserting DNA fragments.
Blue-white screening – A method using the lacZ gene to visually distinguish colonies with or without inserted DNA.
Cosmid – A hybrid vector combining plasmid and bacteriophage λ DNA, used for cloning larger fragments (~45 kb).
BAC (Bacterial Artificial Chromosome) – A low-copy plasmid vector capable of carrying very large DNA inserts (100–300 kb).
Homology-based assembly – Modern cloning techniques (e.g., Gibson assembly, Golden Gate) that join DNA fragments by sequence overlap instead of restriction enzymes.
Sambrook, J., & Russell, D. W. (2001). Molecular Cloning: A Laboratory Manual. 3rd ed. Cold Spring Harbor Laboratory Press.
Watson, J. D., et al. (2014). Molecular Biology of the Gene. 7th ed. Pearson.
Shizuya, H., et al. (1992). Cloning and stable maintenance of 300-kilobase-pair fragments of human DNA in Escherichia coli using an F-factor-based vector. PNAS, 89(18), 8794–8797.
Hartley, J. L., Temple, G. F., & Brasch, M. A. (2000). DNA cloning using in vitro site-specific recombination. Genome Research, 10(11), 1788–1795.
Gibson, D. G., et al. (2009). Enzymatic assembly of DNA molecules up to several hundred kilobases. Nature Methods, 6(5), 343–345.
A cloning vector is designed for propagating DNA fragments — stable storage, replication, and manipulation. An expression vector goes further, containing promoters and other regulatory elements to drive expression of the inserted gene.
Antibiotic resistance allows easy selection of bacteria that carry the plasmid. However, alternatives are being explored to reduce reliance on antibiotics.
Automation enforces SOPs, records digital audit trails, and reduces manual transcription errors—essential for meeting GMP, ISO, or similar standards.
Not as much as in the past. Modern cloning methods like Gibson assembly or Golden Gate allow DNA to be joined without predefined restriction sites, reducing dependence on the MCS.
BACs provide stable maintenance of very large DNA fragments (100–300 kb). They remain valuable for genome assembly, synthetic biology, and cloning viral genomes.
Unlikely. Even synthesized DNA usually needs to be inserted into a vector for stability, amplification, and downstream use. Cloning vectors are still the backbone of gene synthesis workflows.